
Specialist in Clinical Oncology
Clinical Application of Durvalumab in Unresectable Locally Advanced Non-small Cell Lung Cancer
Non-small cell lung cancer (NSCLC) accounts for 85% of all lung cancer cases1. The relatively low rate of survival may due to the fact that the majority of patients are diagnosed with locally advanced or metastatic disease (stage III-IV). Although platinum-based concurrent chemoradiotherapy (CRT) has been the standard of care for patients with unresectable stage III NSCLC in the last three decades, the progression-free survival (PFS) and overall survival (OS) rate remain disappointing2,3. Recently, the revolution of immunotherapy has led to a paradigm shift in lung cancer treatment. In this interview, Dr. Kam shared the latest knowledge of durvalumab, an immune checkpoint inhibitor, and the clinical advice in treating patients with unresectable stage III NSCLC with the use of durvalumab.
Consolidation Therapy with Durvalumab
“A revolutionary treatment of lung cancer has emerged in 2017. It is, durvalumab, a monoclonal antibody against programmed death ligand 1 (PD-L1). Physiologically, PD-L1 binds to its receptor programmed death 1 (PD-1), allowing negative feedback and downregulating immune responses. However, the mechanism could be utilised by cancer cells to escape and suppress the immune system. By inhibiting the PD-L1/PD-1 pathway, durvalumab improves the tumour-mediated immune responses and thus allowing T cells to recognise and kill tumour cells,4” explained Dr. Kam. In the PACIFIC trial, the efficacy of durvalumab was assessed in patients with unresectable stage III NSCLC. A total of 713 patients, who received two or more cycles of platinum-based CRT and did not have disease progression, were randomised to receive durvalumab consolidation therapy or placebo. After a median follow-up of 25.2 months, the median PFS was extended (17.2 vs. 5.6 months) and the risk of disease progression was decreased by 49% in patients who received durvalumab consolidation therapy as compared with placebo5. “With a 1-year durvalumab consolidation therapy following concurrent CRT, the OS rate was impressively improved,” pointed Dr. Kam. Recently, the latest data with 3 years of follow-up has been published. The relative risk of death was reduced by 31% with durvalumab as compared with placebo, and remarkably, more than 50% of patients receiving durvalumab survived at 3 years (57% vs. 43.5% with placebo, Figure 1)6.
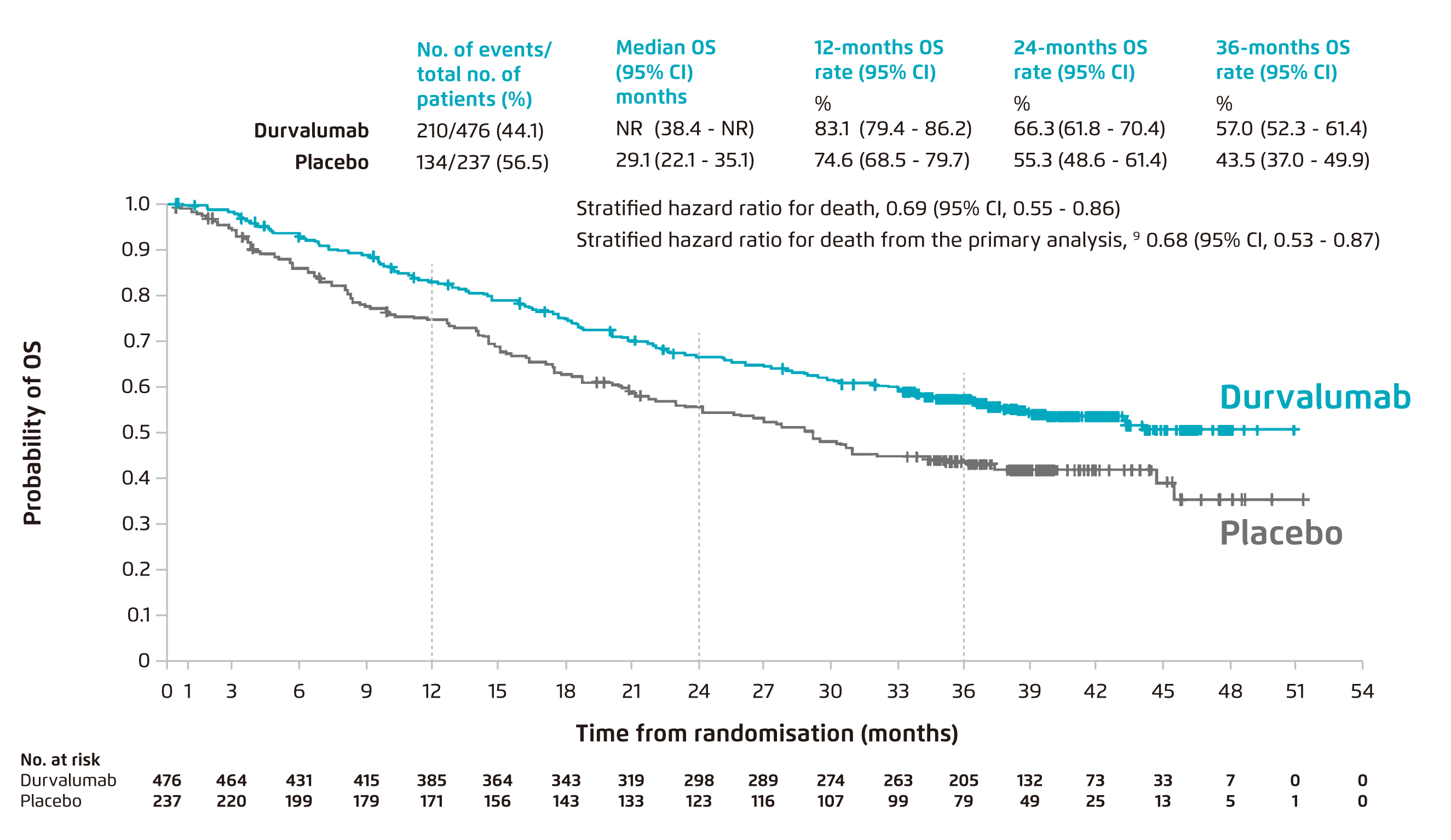
Figure 1. Overall survival in patients treated with durvalumab after concurrent chemoradiotheapy vs placebo, with approximately median 3-year of follow-up6. CI: confidence interval; NR: not reached; OS: overall survival
Less Limitation in Pathological Features
“Although durvalumab is targeting the PD-L1 pathway, the clinical improvements of consolidation therapy are not limited by the expression level of PD-L1 of tumour cells,” added Dr. Kam. The latest subgroup analysis published in March 2020 has revealed that the relative risks of death were decreased by 50%, 11%, and 40% in patients with PD-L1 expression of ≥25% and <25% on tumour cells, and with unknown PD-L1 status, respectively. In addition, the risks of disease progression were also reduced by 41-59% across all prespecified PD-L1 subgroups (Table 1)7. It is noteworthy that the durvalumab consolidation therapy demonstrated clinical effectiveness in patients with both squamous and nonsquamous NSCLC, with the relative risks of disease progression or death reduced by 32% and 55% when compared with placebo respectively8.
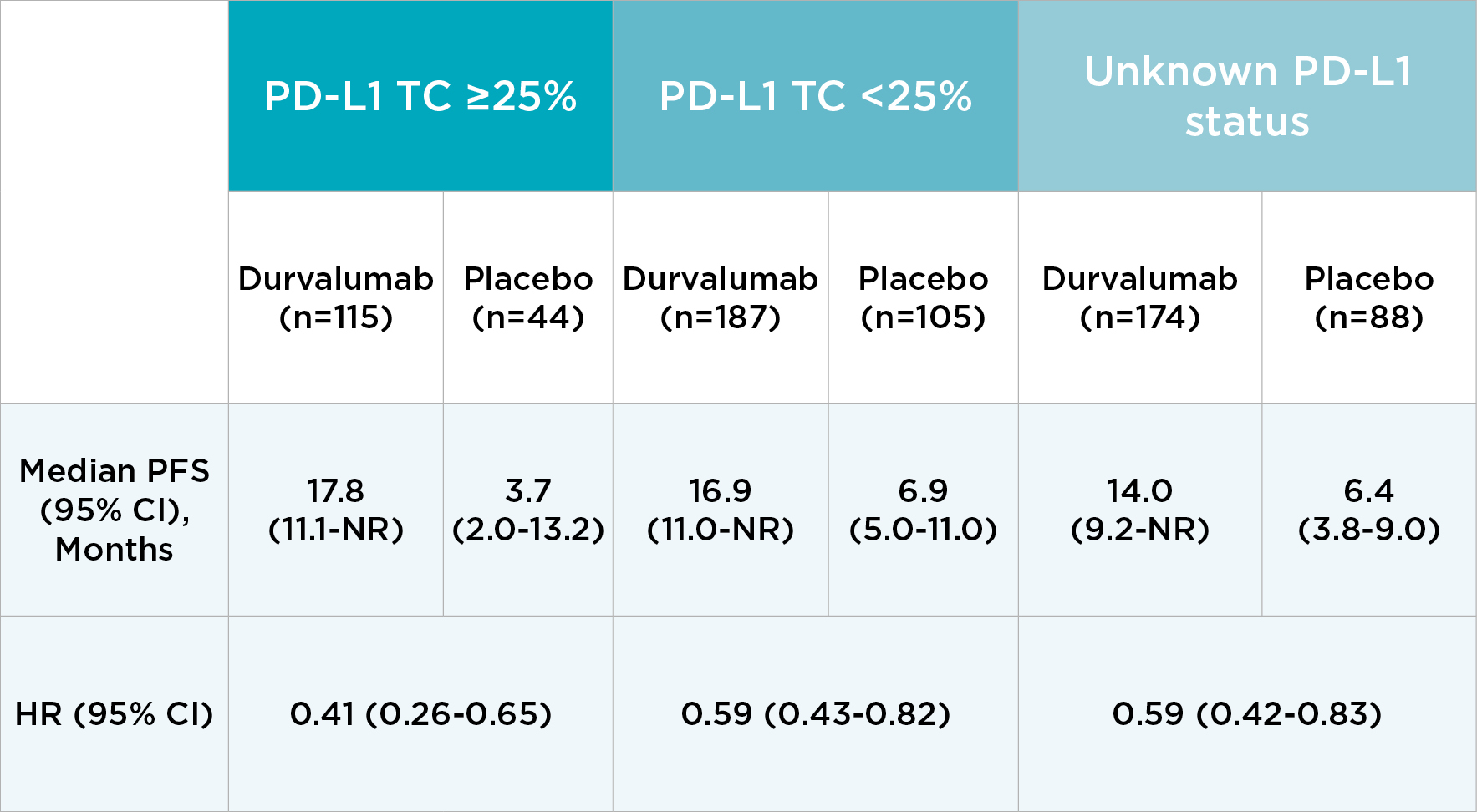
Table 1. Median progression-free survival with durvalumab and placebo by PD-L1 expression level, with 14.5 months of median follow-up7
CI: confidence interval; HR: hazard ratio; NR: not reached; PD-L1: programmed death ligand 1; PFS: progression-free survival; TC: tumour cell
Active Surveillance before Initiation of Durvalumab
“Patients treated with immunotherapy may result in a higher rate of immune-mediated adverse events, including pneumonitis, hypothyroidism, and rashes. However, most are tolerable and manageable,” remarked Dr. Kam. In the PACIFIC trial, grade 3 or 4 adverse events (AE) occurred at a similar rate in both groups of patients, with 30.5% and 26.1% in durvalumab and placebo arms, respectively5. The most frequent AE leading to discontinuation of the treatment were pneumonitis (4.8% and 2.6% in durvalumab and placebo arm, respectively) and radiation pneumonitis (1.3% in both arms)5. “Clinicians should note that this trial may not truly reflect real-world situations because the duration and dose of CRT were strictly controlled in the trial. The risk of pneumonitis after concurrent CRT should be accessed and considered before initiating immunotherapy. In order to minimise the risk of pneumonitis, a break with 3-4 weeks of active surveillance should be offered after the concurrent CRT and before the initiation of durvalumab,” suggested Dr. Kam.
Clinical advice on the use of durvalumab
“As most patients enrolled in the PACIFIC trial did not have epidermal growth factor receptor (EGFR) mutations (~6% of patients in both study arms8), the current data is inconclusive whether durvalumab is beneficial to this subgroup of patients. However, one-year of durvalumab consolidation therapy after concurrent CRT might be considered in this setting because of its impressive improvements in overall survival and duration of response, with approximately 73.5% of patients had an ongoing response at 1.5 year5. For patients with disease recurrence, targeted therapy may be followed,” proposed Dr. Kam. Durvalumab, along with efficacy regardless of PD-L1 expression and favourable effect on metastatic spread prevention7,8, has led to a paradigm shift in the treatment of NSCLC. Dr. Kam advised that consolidation therapy with durvalumab after concurrent CRT is preferred for patients with unresectable stage III NSCLC.
References
1. Zappa C & Mousa SA. Transl Lung Cancer Res. 2016; 5(3): 288-300. 2. Bobbili P, et al. Future Oncol. 2019; 15(29): 3381-3393. 3. Aupérin A, et al. J Clin Oncol. 2010; 28(13): 2181-2190. 4. Soularue E, et al. Gut. 2018; 67(11): 2056-2067. 5. Antonia SJ, et al. N Engl J Med. 2018; 379(24): 2342-2350. 6. Gray JE, et al. J Thorac Oncol. 2020; 15(2): 288-293. 7. Paz-Ares L, et al. Ann Oncol. 2020; S0923-7534(20)36374-2. 8. Antonia SJ, et al. N Engl J Med. 2017; 377(20): 1919-1929.
The article is supported by AstraZeneca





