
Type 2 diabetes mellitus (T2DM) is a persistent state of hyperglycaemia and glucose intolerance that occurs when the body cannot effectively respond to insulin. 14% of adults aged 18 or older were living with diabetes globally in 2022, whereas that was 7% in 19901. While T2DM reduces life expectancy by up to 10 years, the main cause of death for patients with T2DM is cardiovascular disease (CVD)2. In view of the burden of CVD on T2DM patients, clinical guidelines generally advocate glycaemic control as the cornerstone for managing T2DM and as a protective measure against cardiovascular complications3. In recent years, emerging studies on biomarkers and prediction models aiming to estimate the CVD risk among T2DM patients, which facilitate early treatment and optimise outcomes. The purpose of this article is to review the pathophysiology of T2DM complicated by CVD and highlight the clinical management, as well as recent advancements in combating the diseases.
CVD comprises a group of disorders of the heart and blood vessels. Although the lack of glycaemic control is not the only risk factor for CVD, hyperglycaemia may lead to nerve and cardiac conduction impairments and CVD. The incidence of CVD among T2DM patients has been reported to be 2-4 times higher than that of their non-diabetic counterparts4. According to the CAPTURE study (2019), which involved 9,823 T2DM patients across 13 countries, the overall weighted CVD and atherosclerotic CVD (ASCVD) prevalence estimates were 34.8% and 31.8%, respectively. The most prevalent weighted CVD subtypes were coronary heart disease (CHD, 17.7%), followed by carotid artery disease (8.4%), and cerebrovascular disease (7.2%, Figure 1)5.
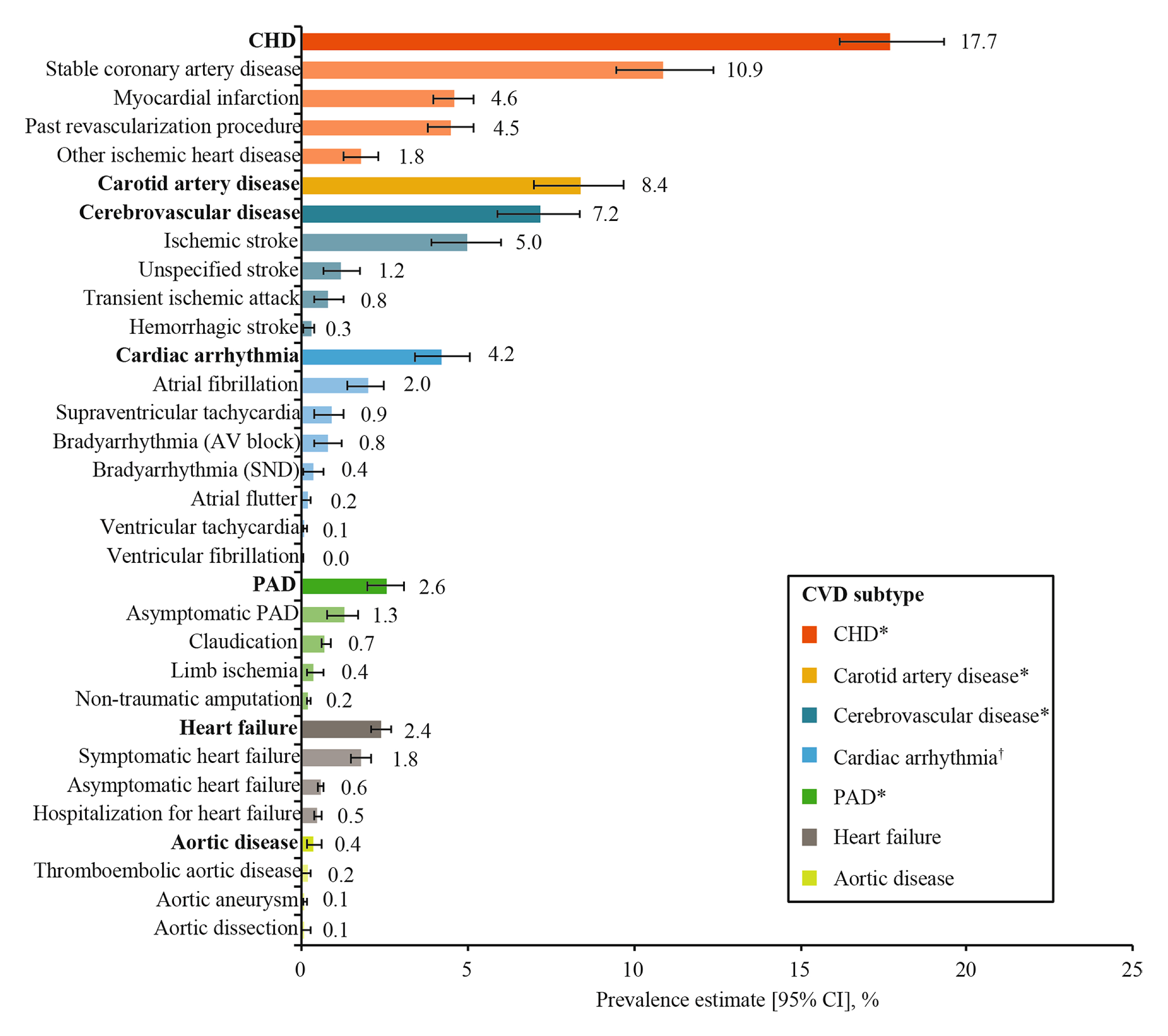
Figure 1. Overall weighted CVD prevalence among T2DM patients5, *Categorised as ASCVD, PAD: peripheral artery disease
Interestingly, a declining trend in CVD incidence among T2DM patients has been consistently reported in various regions since the early 1990s6, particularly in high-income countries, such as the USA7, Hong Kong8 and South Korea9. Nonetheless, there is still a large difference in the incidence of CV morbidity and mortality between T2DM patients and those without the disease.
Regarding the pathophysiological link between CVD and T2DM, various factors that contribute to the development of atherosclerosis and CVD are commonly comorbid in T2DM patients, including hypertension, insulin resistance, hyperglycaemia, obesity, and dyslipidaemia. Atherosclerosis begins with the deposition of lipoproteins in the arterial wall. In the subendothelial space, foam cells accumulate and low-density lipoprotein (LDL) particles are oxidised, leading to vascular modifications. Acute coronary and cerebrovascular syndromes occur when arterial plaque deposits become unstable and rupture. Notably, insulin resistance promotes macrovascular abnormalities through the formation of atheroma plaques, diastolic dysfunction, and ventricular hypertrophy. Moreover, hyperglycaemia promotes the development of CVD through advanced glycosylated end products and oxidative stress among other factors. Both insulin resistance and hyperglycaemia promote coronary artery disease (CAD), cerebrovascular disease, and heart failure10.
Undoubtedly, the disease burden of CVD in T2DM patients is substantial. Apart from the increased morbidity and mortality, a systematic review of 24 articles by Einarson et al. (2018) revealed that CVD costs contributed between 20%-49% of the total direct costs of treating T2DM. The median annual costs per patient for CVD, coronary artery disease, heart failure, and stroke were, respectively, 112%, 107%, 59%, and 322% higher compared with those for T2DM patients without CVD11. The result highlighted the significant increase in the costs attributed to CVD. Given that the burden of CVD in T2DM patients is often avoidable, prevention and early treatment are urgently needed.
It has been widely published that risk factors, including hypertension, dyslipidaemia, obesity, lack of physical activity, poor glycaemic control, and smoking, significantly increase the CVD risk among T2DM patients. For instance, patients with both hypertension and diabetes double their risk for CVD4.
T2DM patients have an increased susceptibility to dyslipidaemia. This correlation is partially due to the enhanced release of free fatty acids (FFAs) in insulin-resistant adipocytes. Elevated FFA levels promote triglyceride (TG) synthesis and, subsequently, lead to the release of apolipoprotein B (apoB) and very low-density lipoprotein (VLDL) cholesterol12. Although the mechanism by which obesity increases CVD risk has not been fully explained, a previous study suggested that each one-unit increase in BMI is associated with a 5% increase in heart failure risk for males and 7% for females, even after adjusting for other heart risk factors13.
Smoking tobacco substantially raises the risk of developing T2DM and its associated complications. Notably, smoking is more strongly linked to CHD risk in females than males, especially among those who smoke over 20 cigarettes daily14.
Importantly, poor glycaemic control appears to be an independent risk factor for all-cause and CVD mortality, independent of other modifiable CVD risk factors in patients with T2DM. Long-term intraindividual variability of HbA1c or basal blood glucose is associated with micro-and macrovascular complications and with an increased risk of important adverse CV events. Moreover, it has been reported that each 1-point increase in HbA1c is associated with an 8% increase in the risk of heart failure and a 36% increased risk for heart failure hospitalisation15.
In patients with T2DM without symptomatic ASCVD or severe target-organ damage (TOD), the 2023 ESC Guideline recommends estimating the 10-year CVD risk using the SCORE2-Diabetes prediction algorithm for T2DM. Similar to the management of most chronic diseases, clinical guidelines generally recommend lifestyle intervention as a core component in preventing CVD risk in T2DM patients. According to the 2023 ESC Guideline, weight reduction and physical exercise are recommended to improve metabolic control and overall CVD risk profile. Regarding smoking cessation recommendations, nicotine replacement therapy, varenicline, and bupropion, as well as individual or telephone counselling, are suggested to improve the smoking cessation success rate3.
Given that reducing HbA1c decreases microvascular complications, particularly when achieving near-normal levels (HbA1c<7%, <53 mmol/mol), the 2023 ESC Guideline recommends applying tight glycaemic control to reduce microvascular complications, whereas the HbA1c targets should be individualised according to the patient's comorbidities, diabetes duration, and life expectancy3.
Regarding pharmacotherapy, metformin remains the first-line treatment, as per the 2023 ESC Guideline. The therapy is continued until intolerance or the occurrence of ASCVD or indicators of high ASCVD risk. For patients with SCORE2-Diabetes reaches 10%, (sodium-glucose cotransporter 2) SGLT-2 inhibitor or (glucagon-like peptide 1 receptor agonist) GLP-1RA should be considered (Figure 2)3.
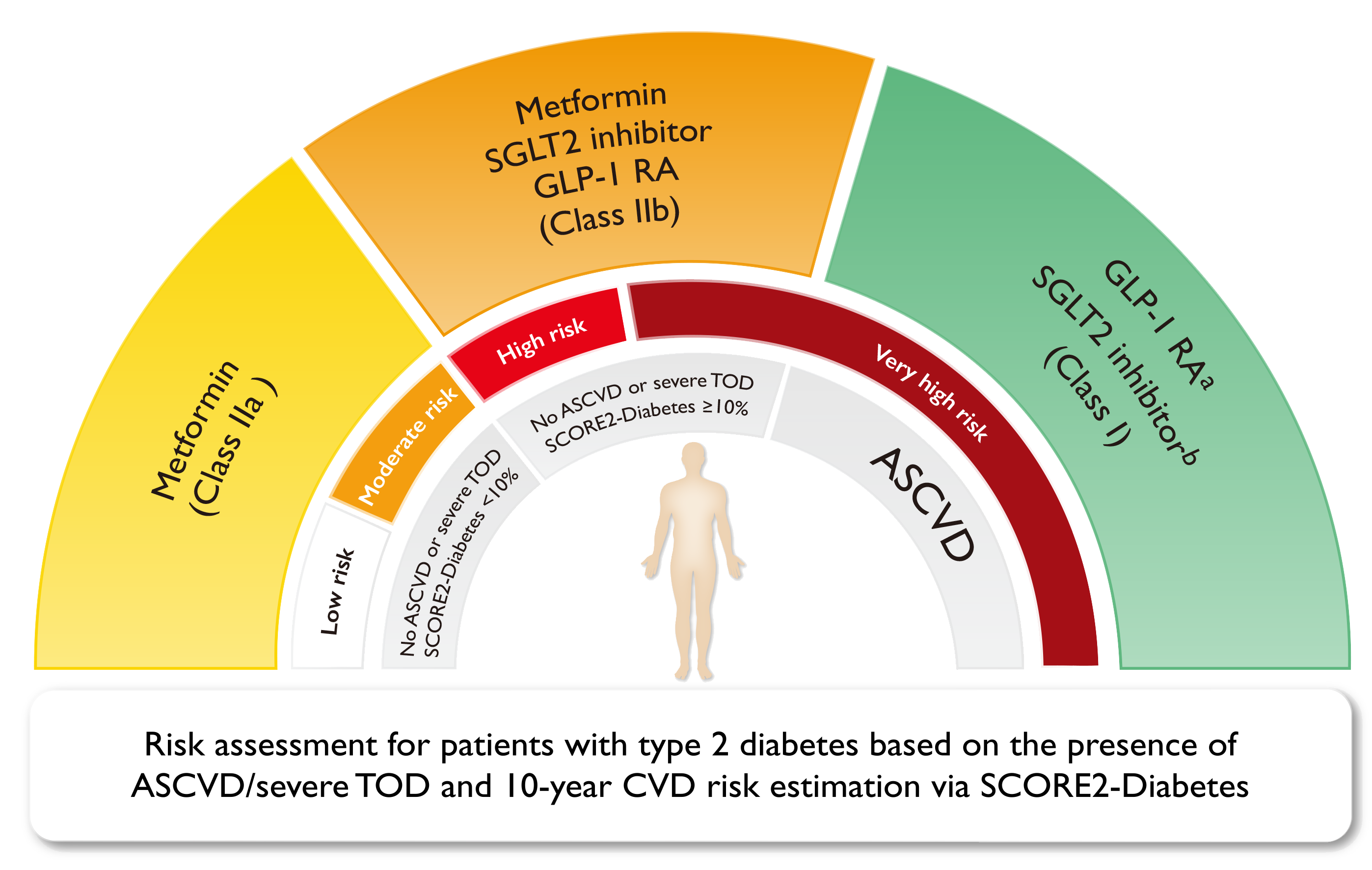
Figure 2. Glucose-lowering treatment for T2DM patients to reduce CV risk3
Besides glycaemic control, high levels of LDL-C and non-high-density lipoprotein cholesterol (non-HDL-C) and low levels of HDL-C are associated with an increased risk of CV events and mortality in patients with and without diabetes. Thus, lipid-lowering agents are also recommended for T2DM patients with dyslipidaemia. The 2023 ESC Guideline recommends target LDL-C levels of <1.8 mmol/L and <1.4 mmol/L for high-risk and very high-risk patients, respectively. Statins are recommended as the first-line therapy to reduce LDL-C levels due to their efficacy in preventing CV events and reducing CV mortality3.
While CVD remains a leading cause of morbidity and mortality in T2DM patients, there are emerging investigations aiming to identify biomarkers of CVD in order to improve targeted therapies for the prevention and treatment of CVD among these patients. Currently, most candidate biomarker studies focus on known pathophysiological pathways, such as those related to cardiac stress, inflammation, matrix remodelling, lipids, endothelial dysfunction, and diabetes pathophysiology16.
In a recent study by Meng et al. (2024), 900 Chinese people were recruited and, according to their medical history, were allocated into 3 groups, namely healthy control group (HC, n=300), new-onset T2DM group (T2DM, n=300), and new-onset T2DM with CHD group (T2DM+CHD, n=300). In each group, the participants were further categorised into 3 groups based on their age. Fasting glucose, HbA1c, triglycerides, total cholesterol, LDL-C, HDL-C, apolipoprotein A1 (ApoA1), ApoB, ApoB/ApoA1 ratio, lipoprotein(a) [Lp(a)], high-sensitivity C-reactive protein (hsCRP), and homocysteine (HCY) levels were analysed in all groups17.
Although existing evidence generally accepts that dyslipidaemia is associated with an increased risk of CVD in T2DM patients, the results did not show a significant difference in LDL-C, triglycerides, and total cholesterol levels between T2DM and T2DM+CHD groups. Instead, the results indicated significant increases in ApoB, Lp(a), and hsCRP levels in the T2DM+CHD group compared to the T2DM group, whereas other biomarkers did not show significant differences (Figure 3A-3C). Across all age groups, the patterns remained consistent17. Given that Lp(a) and hsCRP levels showed particularly notable elevations, the biomarkers are potential indicators of CHD risk in the T2DM population.
 and high-sensitivity C-reactive protein are predictive biomarkers-4.jpg)
Figure 3. Biomarker levels in HC, T2DM, and T2DM+CHD groups17, *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001
Apart from investigating the associations of single biomarkers with CVD risk in T2DM, there are studies evaluating a large number of biomarkers simultaneously. For instance, in the study by Gerstein et al. (2015), a set of 237 biomarkers from 8,401 participants with dysglycaemia were analysed. Biomarkers that were each independent determinants of 3 different incident outcomes, namely (1) the composite of myocardial infarction, stroke, or CV death, (2) these plus heart failure hospitalisation or revascularisation, and (3) all-cause death, were identified. Remarkably, 3 biomarkers (NT-proBNP, angiopoietin 2, and glutathione S transferase α) were consistently identified in all of the Cox regression models and for all of the outcomes, with NT-proBNP being the strongest predictor of all 3 outcomes18.
In the era of artificial intelligence (AI), AI-based technology has been applied to optimise the prediction of CVD risks among T2DM patients. For instance, Ding et al. (2023) developed machine learning models to predict the 3-year ASCVD risk in T2DM patients based on the clinical data of 4,722 T2DM patients, which included demographic information, disease histories, laboratory tests and physical examinations. 5 machine learning methods (logistic regression [LR], support vector machine [SVM], gradient boosting decision tree [GBDT], random forest [RF], and adaptive boosting [AdaBoost]) were developed, with good performance in both internal and external test sets (Figure 4)19. The study served as a promising demonstration of applying machine learning technology to develop prediction models for personalising treatment and optimising outcomes.
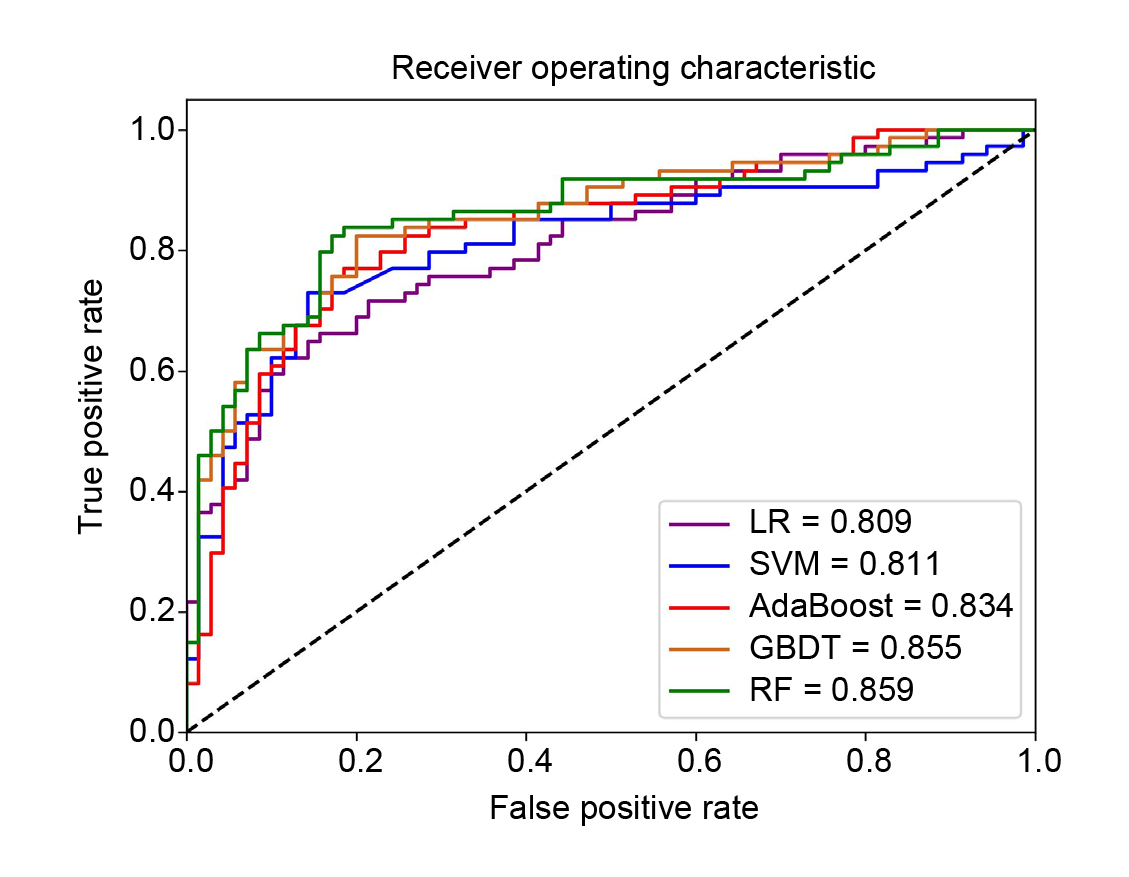
Figure 4. Receiver operating curves for the prediction of ASCVD using different machine learning models in the testing set19
Clinicians have widely recognised the pathological linkage between CVD and T2DM. Hence, treatment recommendations for preventing and controlling CVD among T2DM patients have been addressed in clinical guidelines. Nonetheless, early identification of T2DM patients with a high risk of CVD remains a challenging clinical issue. Even worse is that T2DM patients are often at risk of other complications in addition to CVD, such as chronic kidney disease (CKD) in the case known as Cardiovascular-Kidney-Metabolic (CKM) syndrome. The complications coexist and exacerbate each other, leading to a higher risk of adverse health outcomes and increasing the difficulty in treating the patients. However, with the better understanding of the pathophysiology of T2DM and its complications, and the development of advanced technologies, particularly AI, more accurate prediction models and effective.
References
1. World Health Organization. Diabetes. https://www.who.int/news-room/fact-sheets/detail/diabetes. 2. International Diabetes Federation. Diabetes & Heart. 2016. https://idf.org/about-diabetes/diabetes-complications/cardiovascular-disease/. 3. Marx et al. Eur Heart J 2023; 44: 4043–140. 4. Hudspeth. Am J Manag Care 2018; 24: S268–72. 5. Mosenzon et al. Cardiovasc Diabetol 2021; 20. DOI:10.1186/S12933-021-01344-0,. 6. Yun and Ko. Metabolism 2021; 123. DOI:10.1016/J.METABOL.2021.154838/ASSET/104A0D2F-9D5A-4DEF-A5EE-0AA261C8FF92/MAIN.ASSETS/GR1.JPG. 7. Gregg et al. The Lancet 2018; 391: 2430–40. 8. Luk et al. Diabetes Care 2017; 40: 928–35. 9. Park et al. Diabetes Metab J 2020; 45: 120–4. 10. Joseph et al. Circulation 2022; 145: 722–59. 11. Einarson et al. Value in Health 2018; 21: 881–90. 12. Siam et al. Rev Cardiovasc Med 2024; 25. DOI:10.31083/J.RCM2512436/219035EB3A5B7D54827006F55360A8D0.PDF. 13. Welsh et al. Eur J Prev Cardiol 2024; 31: 1026–35. 14. Balakumar et al. Pharmacol Res 2016; 113: 600–9. 15. Roman et al. Type 2 Diabetes - From Pathophysiology to Cyber Systems 2021; published online April 20. DOI:10.5772/INTECHOPEN.97422. 16. Bachmann and Wang. Diabetologia 2017; 61: 987. 17. Meng et al. Heliyon 2024; 10. DOI:10.1016/j.heliyon.2024.e40074. 18. Gerstein et al. Circulation 2015; 132: 2297–304. 19. Ding et al. J Diabetes Investig 2023; 14: 1289–302.





