

Editor-in-Chief, V·Pulse

Doctor at the National Health Services (NHS), United Kingdom (UK)
Precision oncology (PO), defined as molecular profiling of tumours to identify targetable alteration1. The approach has shifted away from non-specific cytotoxic treatment to a more individualised cancer treatment. The advancement in diagnostic technologies, such as next-generation sequencing (NGS), with decision support applications and the availability of patient databases has facilitated optimal cancer management. While PO has proven successful in improving patient outcomes and quality of life (QoL), challenges still persist due to hinderance its use for matching therapies to the patients. Fortunately, emerging techniques, including artificial intelligence (AI), have been implemented to enhance the clinical performance of PO. This review aimed to outline some of the practical issues related to PO and its recent development.
PO involves the detection of tumour-specific aberrations and combating them with drugs that targets cells with the altered genomic status2. In fact, the concept of PO is not new since the BCR-ABL rearrangement in chronic myeloid leukaemia (CML) was identified and targeted by imatinib in 1998, which led to dramatic clinical remissions. Accordingly, the therapy was approved by the U.S. Food and Drug Administration (FDA) in 20011.
With the development of sophisticated “omic” methods, profiling of the complex molecular characteristics of tumours, such as DNA sequence data (genomics), RNA analysis (transcriptomics), protein levels (proteomics), cellular metabolism (metabolomics), etc, has become readily available3. The discovery of actionable mutations and biomarker profiling not only improves our understanding of cancer biology but also helps to optimise clinical management of oncology patients.
Analysis of nationwide database in the United States (U.S.) by Steuten et al. (2019) involving 5,688 patients with advanced non–small-cell lung cancer (NSCLC), who received multigene panel sequencing (MGPS, n=875) or single-marker genetic testing (SMGT, n=4,813), revealed that 30.1% of MGPS-tested patients had evidence of an actionable mutation, such as epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK), and 21.4% received a targeted treatment, whereas actionable mutation was found in 23.3% of SMGT-tested patients and 18.7% received a targeted treatment (Figure 1). Essentially, the results suggested that patients who received targeted treatments for specific mutations exhibited better overall survival (OS) compared to those who did not receive a targeted treatment (mean adjusted OS: 2.31 years [targeted treatment] vs 1.73 years [non-targeted treatment])4.
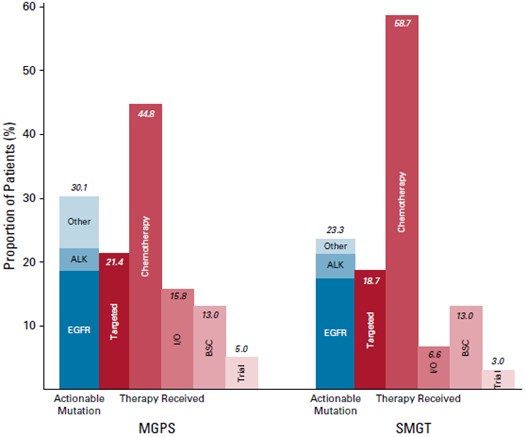
Figure 1. The proportion of patients with an actionable mutation and type of therapy received4
Therefore, using biomarker profiling to guide cancer treatment likely improves the therapeutic outcomes for cancer patients. Remarkably, the advent of NGS on formalin-fixed, paraffin-embedded tissues allows the determination of mutations in a large number of genes in a timely and cost-effective manner.
NGS is a technology for deoxyribonucleic acid/ribonucleic acid (DNA/RNA) sequencing and mutation detection. Practically, the major steps involved in NGS include DNA/RNA fragmentation, library preparation, massive parallel sequencing, bioinformatics analysis, and mutation annotation and interpretation (Figure 2)5. Briefly, DNA fragmentation is the process of breaking down targeted DNA into segments of 100-300 base pair (bp) in length. Relevant segments are then extracted by hybridisation capture assay or polymerase chain reaction (PCR) amplification. Library preparation is a process by which DNA segments are modified so that each DNA sample can have a sample-specific index. It also allows the sequencing adaptors to be added to the DNA segments. The DNA segments are then proceeded to massive parallel sequencing using an NGS sequencer. Subsequently, the sequence information generated from massive parallel sequencing is analysed using bioinformatics software6.
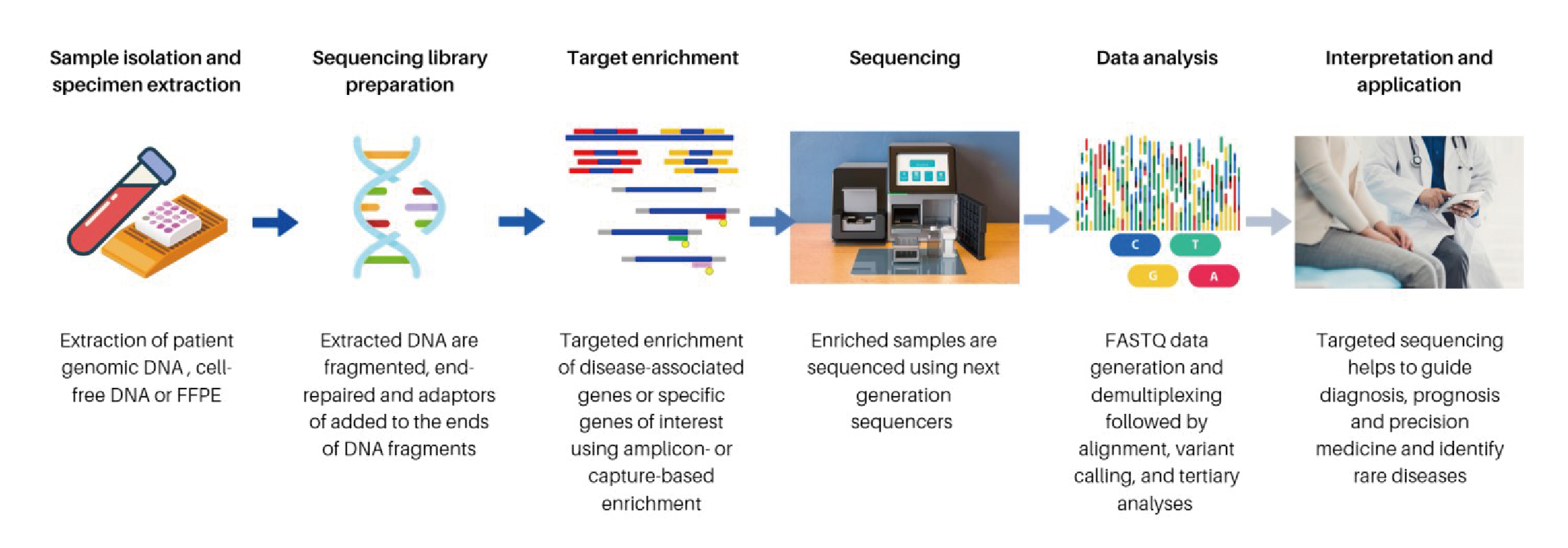
Figure 2. General workflow of NGS5
NGS enables the rapid, cost-effective detection of a broad spectrum of well-characterised genomic alterations, including short structural variants (SSVs), copy number alterations (CNAs), translocations, and fusions in multiple genes. The technology drives the clinical application of PO due to the rapid expansion of treatable gene aberrations and predictive biomarkers in recent years, especially in lung cancer and gynaecological oncology. For instance, the recent data from the United Kingdom (U.K.) 100,000 Genomes Project provided whole-genome sequencing information for 13,880 solid tumours. Interestingly, the findings revealed clinically relevant mutations were present in 20-49% of gynaecological cancers, including invasive breast carcinoma, high-grade ovarian serous carcinoma, and uterine endometrial carcinoma7. Hence, large-scale tumour molecular profiling programs using NGS have fostered the growth of precision cancer medicine, while NGS-based molecular pathology has become an essential tool in driving therapeutic decision-making.
The advent of PO has changed the landscape of oncologic biomarkers, drug discovery, and outcomes for patients with cancer. For most cancer types, the one-size-fits-all treatment approach is fast becoming obsolete, whereas the PO approach, which treats the right patient with the right treatment at the right time, has rapidly entered mainstream clinical practice. The PO approach to cancer treatment aims to maximise clinical efficacy, minimise safety concerns, and reduce economic burden.
While NGS cancer profiling has gained traction in routine clinical practice in South Korea, a recent analysis of real-world data of 990 patients with advanced solid tumours in tertiary hospitals by Kim et al. (2025) evaluated the outcomes of NGS testing and genomically matched therapies. Among patients with variants of strong clinical significance such as FDA-approved, professional guidelines, or well-powered research-based therapy (tier I variants), the most frequently altered genes detected were Kirsten rat sarcoma virus (KRAS) (10.7%), followed by EGFR (2.7%) and V-Raf Murine Sarcoma Viral Oncogene Homolog B (BRAF) (1.7%, Figure 3). Among patients with measurable lesions who received NGS-based therapy (n=32), 37.5% achieved a partial response, and 34.4% achieved the stable disease. The results further suggested that NGS-based therapy resulted in a higher response rate (37.5%) than standard of care. The median treatment duration was 6.4 months, and the median OS was not reached8. The findings demonstrated that the PO approach with NGS profiling was successfully implemented in daily clinical practice for patients with advanced malignancies and led to improved treatment outcomes.
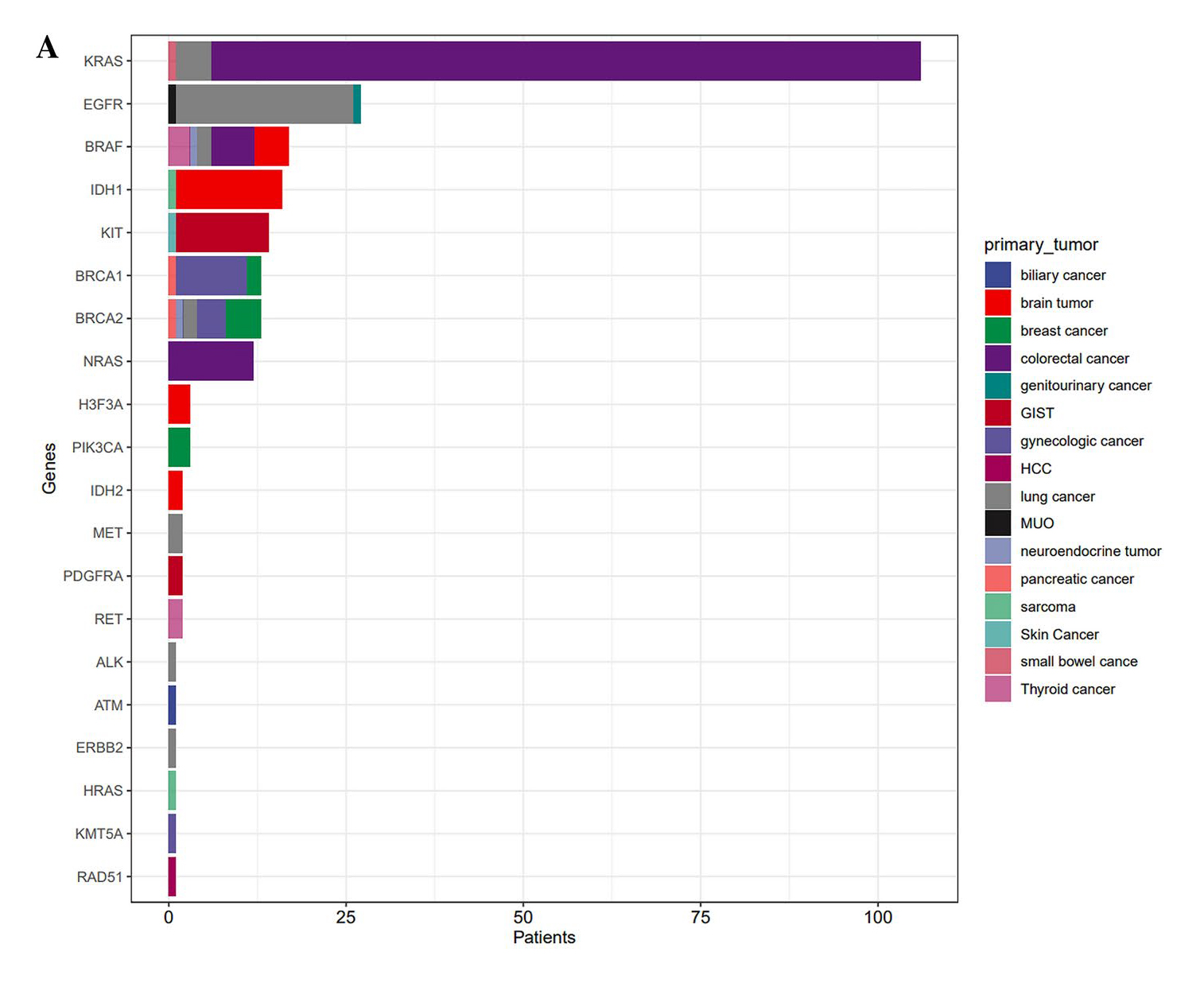
Figure 3. Genetic alterations identified using NGS profiling in South Korea real-world study8
On the other hand, an analysis by Lau et al. (2024) evaluated the response and survival of precision-guided treatment (PGT) among 384 high-risk paediatric cancer patients. A total of 256 (67%) patients received PGT recommendations, and 110 (29%) received a recommended treatment. The types of targeted therapy in relation to the drug target are summarised in Figure 4. The result indicated that PGT yielded a 3-year OS of 34% in the 384-patient cohort. Remarkably, PGT achieved a 36% objective response rate (ORR) (complete response [CR]: 9% and partial response [PR]: 27%) and stable disease in 34% of patients. Moreover, PGT also improved 2-year progression-free survival (PFS) compared with standard of care (SOC) (26% vs 12%, p=0.049, Figure 5A) or targeted agents not guided by molecular findings (UGT) (26% vs 5.2%, p=0.003, Figure 5B)9. The results confirmed that PGT informed by molecular profiling significantly improves outcomes for children with high-risk cancers.
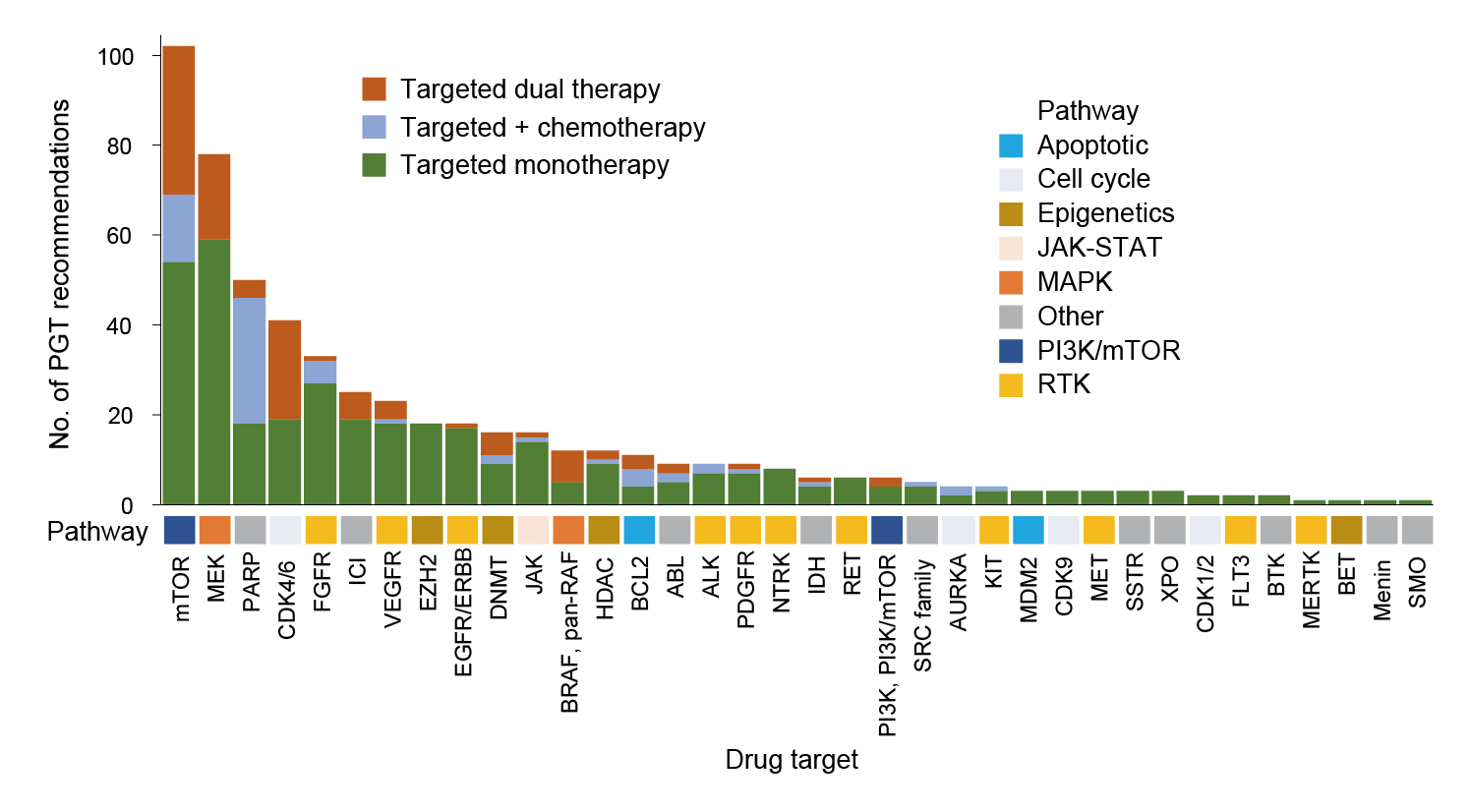
Figure 4. Types of targeted therapy in relation to the drug target9
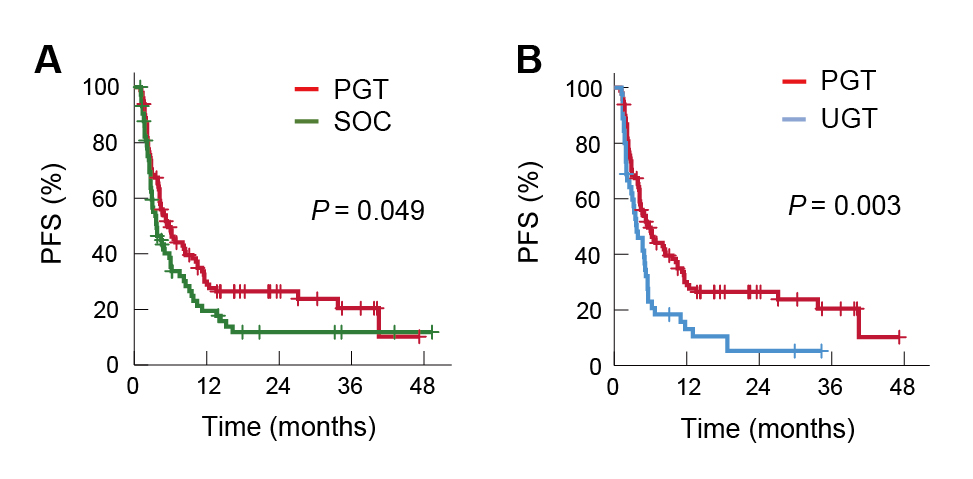
Figure 5. Improved PFS achieved by PGT treatment against A) SOC and B) UGT9
The survival benefits of the PO approach in patients with end-stage cancers were demonstrated in the prospective study by Mapendano et al. (2025). 196 patients with end-stage cancers at a single oncological centre were included, and whole exome and RNA sequencing were performed based on the targeted treatment prescribed. A driver variant was identified in all but 3 patients, whereas 42% were affected simultaneously by more than oncogenic pathways. Notably, druggable targets were suggested in 42% of patients, but two-thirds were not treated. For patients treated (n=30), the clinical benefit rate (CBR) was 44%, and the median time on treatment was 3.5 months. Essentially, the median OS was about 2.6 times longer for patients with a recommended targeted treatment initiated (15.7 months) as compared with patients with no druggable targets (6.5 months, p=0.003) and those who were not treated by targeted drugs (5.8 months, p=0.004, Figure 6). The report suggested that targeted treatment was the strongest prognostic predictor10.
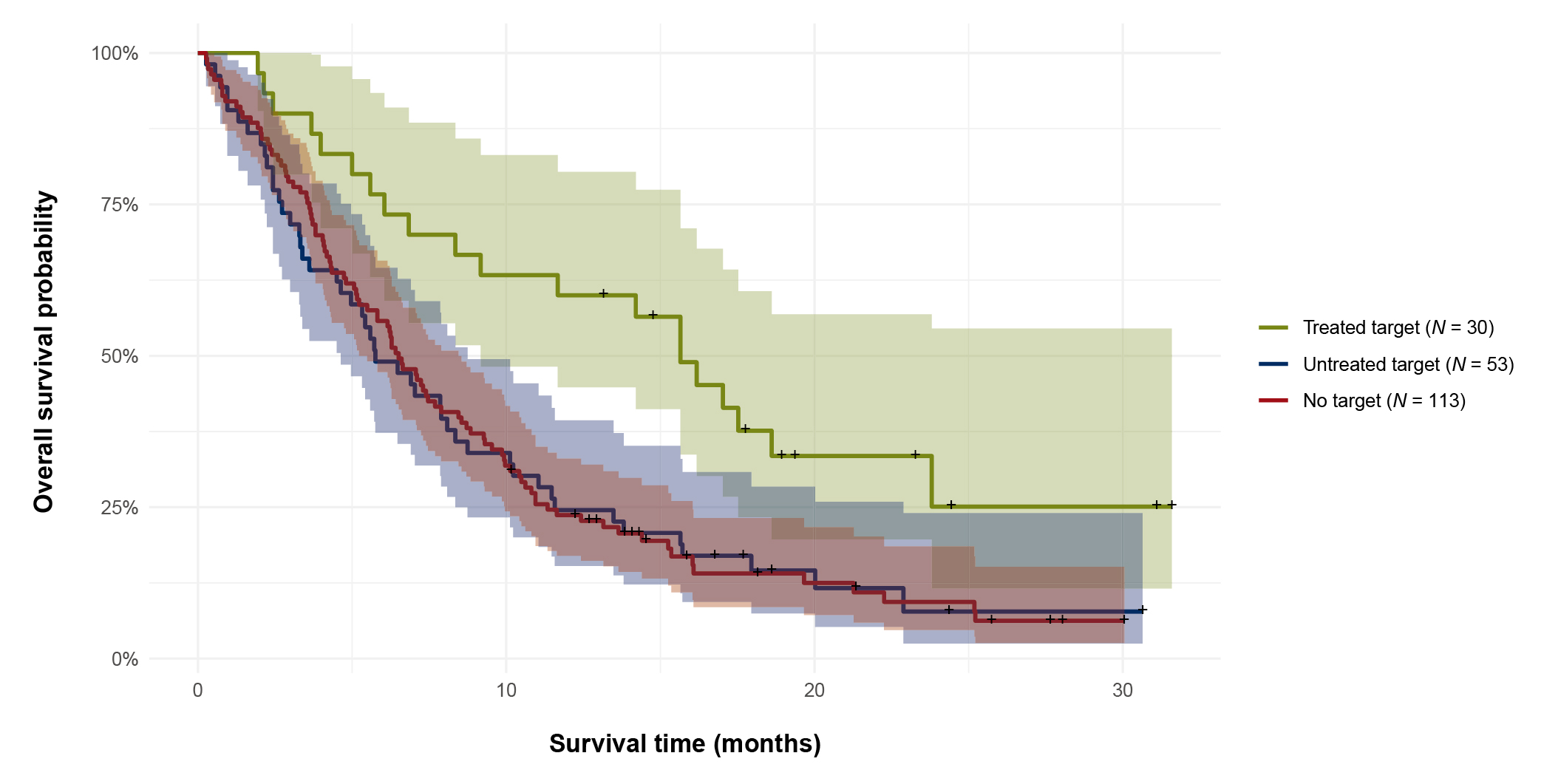
Figure 6. OS of end-stage cancer patients according to targeted treatment groups10
Published clinical data showed that the PO approach yielded significantly better survival benefits compared to the standard treatment; however, PO approach appeared to be feasible in only a small proportion of patients.
Undoubtedly, PO approach has revolutionised the landscape of cancer management; however, there are obstacles hindering the clinical applications of PO. For instance, only a small number of patients with specific cancer types may benefit since targeted therapies against certain biomarkers remain unavailable3. In 2018, one-third of all human proteins are yet to be studied in detail, and only 3% were targets of at least one approved drug with a known mechanism of action11. In this regard, novel approaches to drug development, such as multifunctional drugs, gene silencing, genetic modification, disruption of the target interactome, target degradation, synthetic lethality, or targeted activation of an antitumor immune response, may be effective3.
Besides, translating the benefits of PO into reality for improving healthcare outcomes is heavily dependent on our ability to identify disease or drug-associated actionable genetic mutations. Accordingly, big data analysis of diverse genomic data and electronic health records provides an efficient and effective way to identify clinically actionable genetic variants for personalised treatments12.
While NGS provide comprehensive tumour profiles of patients, the cost and throughput advantages of NGS are offset by large trade-offs concerning short sequencing read length and accuracy. Particularly, its high error rate makes it extremely difficult to detect single nucleotide polymorphisms (SNPs) or low-abundance mutations. In this regard, it has been suggested that the dual-base sequencing strategy, where each sequencing run provides only an ambiguous sequence with a partially defined base composition, can provide an inherent proofreading function, thereby reducing errors in the original data13.
Moreover, interpreting NGS test results remains challenging in practice, making it difficult to administer genomically matched therapies. Remarkably, restrictions on the off-label use of matched drugs may also limit the use of PO-recommended treatments.
The PO approach has revolutionised traditional cancer management, and the “one-size-fits-all” cytotoxic treatment is being rapidly replaced by individualised treatment. Emerging clinical investigations demonstrated the improvement in patient outcomes achieved by the PO approach; nonetheless, only a handful number of cancer patients currently benefit from the PO approach due to various technical obstacles. Fortunately, multidisciplinary efforts have been made to optimise PO in clinical settings, particularly with the artificial intelligence (AI), which is anticipated to help overcome these shortfalls.
While PO requires the molecular profiling of tumours to identify targetable alterations, AI allows the integration of vast data derived from multi-omics analyses. Moreover, AI is expected to play a crucial role in future image analysis in radiological oncology. The technology would enhance the accuracy and efficiency from upstream image reconstruction, registration, segmentation, disease diagnosis, and plan optimisation to therapy prognosis and prediction14.
Apart from diagnostic procedures, AI is expected to facilitate the development of new biomarkers for precise disease characterisation by allowing the processing of vast, heterogeneous datasets to identify variables most strongly correlated with specific clinical outcomes. These variables are then integrated into predictive algorithms for use with new patient data15.
Although the application of AI to PO is still in the early phase, emerging literature has provided an insight into its future applications in cancer management. The dawn of treatment enhancement with PO is emerging and, more importantly, this improvement will help make a difference to the lives of oncology and non-oncology patients in near future.
References
1. Schwartzberg et al. American Society of Clinical Oncology Educational Book 2017; 160–9. 2. Ding et al. Mol Cancer Res 2018; 16: 269–78. 3. Rulten et al. Int J Mol Sci 2023; 24. DOI:10.3390/IJMS241612613. 4. Steuten et al. JCO Clin Cancer Inform 2019; 3: 1–10. 5. Pei et al. Cells 2023, Vol 12, Page 493 2023; 12: 493. 6. Qin. Cancer Biol Med 2019; 16: 4. 7. Gremke et al. Cancers (Basel) 2024; 16: 1561. 8. Kim et al. Scientific Reports 2025 15:1 2025; 15: 1–14. 9. Lau et al. Nature Medicine 2024 30:7 2024; 30: 1913–22. 10. Mapendano et al. ESMO Open 2025; 10: 104089. 11. Oprea et al. Nat Rev Drug Discov 2018; 17: 317–32. 12. Carter et al. J Healthc Eng 2016; 2016. DOI:10.1155/2016/3617572. 13. Cheng et al. Front Bioeng Biotechnol 2023; 11: 982111. 14. Cui et al. Br J Radiol 2023; 96: 20230142. 15. McGale et al. Oncotarget 2024; 15: 588.





