
Botulinum toxin (BoNT-A), also known as Botox, is a highly potent neurotoxin or as some consider neuromodulator, produced by the bacterium Clostridium botulinum, a spore-forming, gram-positive anaerobic bacteria1. Clostridium botulinum is capable of procedure at least seven different serotypes A-G neurotoxins, with serotype A being most common neurotoxin used for cosmetic purposes. Botox use initially started in early 1970s in the field of ophthalmology and eventually expanded into different aspects of health, particularly in dermatology over the last 20 years or so2,3. Onabotulinum toxin A was the first type of Botox introduce to the cosmetic marker which was approved by the Food and Drug Administration (FDA) in 2002 for glabellar frown line treatment, with subsequent formulation approved for different cosmetic treatment in 2006 and 2009, respectively2,4. We may then ask, is Botox primarily used for cosmetic purposes and how does it work? The answer to first part of the question is no, even though Botox is often associated to cosmetic treatment, it is also used to treat a number of medical conditions which include chronic migraine, cervical dystonia, strabismus, hyperhidrosis, urinary incontinence for detrusor overactivity, and hemifacial spasm5.
The toxin works by diffusing into tissues until it binds selectively and reversibly to the presynaptic terminals of the neuromuscular junction. Upon binding, it attaches to the specific protein-membrane responsible for acetylcholine (ACh) excretion and prevent the release of this neurotransmitter, thereby reducing the facial wrinkles as illustrated in Figure 12. As interesting as it sounds, Botox use should be avoid in those with myasthenia gravis, amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), Eaton Lambert syndrome, pregnant females, and those breast feeding, in addition to neonates and those with hypersensitivity2. But are the effects of Botox permanent and how long do they take to wear off? The clinical effects of Botox are not permanent, and the effects of the neurotoxin are obvious between 2-4 days after injection, and may takes up to 3-4 weeks to reach its full potential; these effects eventually wears out after 3-4 months6. Furthermore, the efficacy of Botox is limited, particularly for treating eyebrow asymmetry since Botox injection is unable to accurately target deeper muscle bellies that are required for correcting the eyebrow asymmetry7.
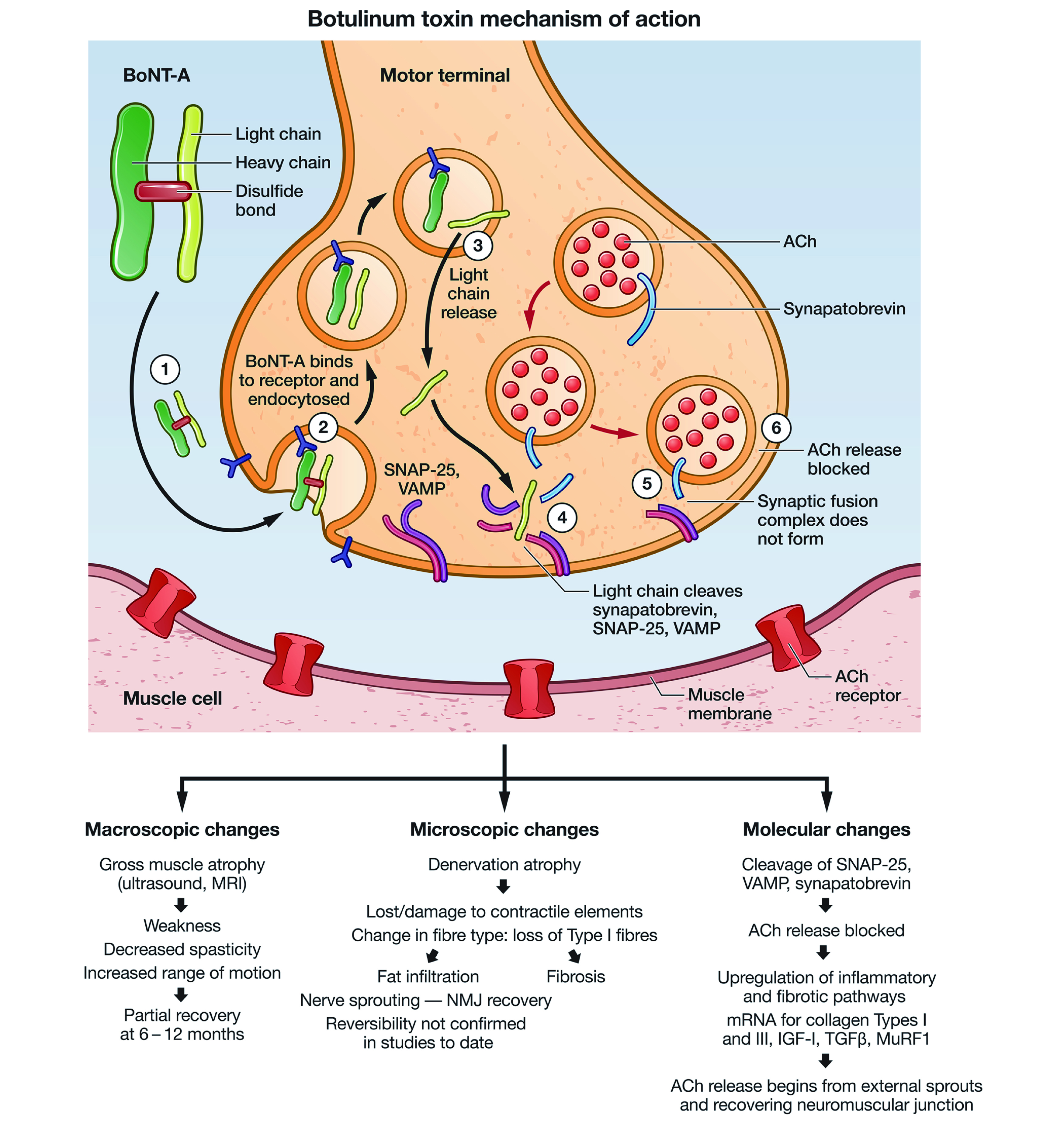
Figure 1. Botulinum toxin type A (BoNT-A) heavy and light chain lined by disulphide bonds. Acetylcholine (ACh), the neurotransmitter is blocked by the BoNT-A results in the muscle paralysis. SNAP 25 (soluble N-ethylmaleimide fusion protein), VAMP (vesicle associated membrane protein)8.
The effects of Botox are not prevalent in some individuals, known as “non-responders”. In fact, some develop resistance to the Botox treatment, thereby, limiting its future therapeutic benefit. What is the aetiology behind this resistance? It is believed by some that Botox treatment resistance is mediated by neutralizing antibodies (nABs) or through non-immunogenic cause such as poor handling of products, inappropriate technique or dosage use during treatment9,10. The rationale behind the nABs is that repeated Botox injections and higher doses of BoNT-A may provoke immune reaction which then produces nABs that reduces or diminishes the effects of BoNT-A, referred to as secondary nonresponse (SNR)11. It is natural to ask if any Botox alternative exists and whether they are equally as effective as Botox or better.
The answer to this is, Yes and DaxibotulinumtoxinA, also known as DaxibotulinumtoxinA for Injection (DAXI), is the new alternative to BoNT-A recently approved by the FDA. DAXI is a type of botulinum toxin type A, which is highly purified, with 150 kilodalton (kDa) core with proprietary stabilising excipient peptide, also known as RTP004. So, what is so special about RTP004? RTP004 is positively charged, which enhances the binding capabilities of DAXI to the neuronal surfaces, thereby improving neurotoxin internalisation12. The 36-week efficacy data obtained from Phase 3 SAKURA clinical trial on the effects of DAXI showed the efficacy of DAXI lasts for ≥24 weeks compared to BoNT-A treatment13. Furthermore, patients with eyebrow asymmetry responded well to DAXI injections compared to BoNT-A injections as per the very recent study14.
When was DAXI approved by the FDA? Are there any safety concerns? DAXI was approved by the FDA on 8th September 2022, licensed as a nicotinic acetylcholine receptor antagonist (nAChR) antagonist/Synaptosomal-Associated Protein, 25kDa (SNAP-25) inhibitor15. In addition, the adverse effects (AEs) reported are mostly mild in nature which often resolves after a short period16. So, is DAXI the holy grail for anti-wrinkle treatment? Unfortunately, it is too early to say since the clinical data on DAXI use for anti-wrinkle treatment is limited and DAXI is still not available commercially in some countries. Furthermore, DAXI is bound to face similar fate to BoNT-A in terms of its long-term efficacy, but here some may argue, why not simply increase the drug dose to prolong its effect and duration? Studies on onaboutlinum toxin A showed increasing the dose to 20 units above FDA recommended dose shows no meaningful improvement in duration with no statistical differences in terms of efficacy compared to standard recommended dosing17.
The promising results of DAXI were obtained from two randomised, double-blinded, multi-center clinical trials, the studies SAKURA 1 (GL-1) and SAKURA 2 (GL-2). Both trials evaluated DAXI use in moderate-to-severe glabellar line in adults13. Moreover, the trials enrolled a total of 609 subjects (≥18 years old) with glabellar lines of at least moderate severity were assessed using the Investigator Global Assessment-Frown Wrinkle Severity Scale and the Patient Frown Wrinkle Severity Scale, respectively. A total of 405 subjects were treated with 40 units of DAXI and 204 subjects were treated with placebo. The treatment success was reported in 74% of DAXI cohorts compared to 0% of placebo cohorts13. Similar efficacy was reported in a Phase 2 study conducted by Dover et al., 2022 with DAXI administered at different doses (40U [glabellar], 32U [forehead], and 48U [lateral canthal lines]). in addition to achieving a high response rate for extended duration and high patient satisfaction. Furthermore, none of the treatment groups experienced eyebrow or eyelid ptosis18.
Nonetheless, some may then ask what are the differences between DAXI and BoNT-A? The primary differences between DAXI and BoNT-A is the rate at which DAXI work. Studies have shown that the DAXI works much faster than BoNT-A and the median duration of efficacy of DAXI is approximately 24 weeks compared to 3 months lifeline of BoNT-A12. Furthermore, BoNT-A requires neurotoxin associated proteins (NAPs) which are composed of three haemagglutinins and one non-toxic, non-haemagglutinin protein. These NAPs protect the BoNT-A against change in pH and helps to hold molecules in place. Unlike BoNT, DAXI does not utilise NAPs, instead it is coated with peptides which helps the neurotoxin bind quickly and for a longer period. In addition, DAXI is considered by some as a “pure toxin” since it does not use any NAPs, thereby, the likelihood of developing antibodies against it is lower; this then translates to longer therapeutic effect as illustrated in Figure 2 and 3 19. Apart from being used as anti-wrinkle treatment, DAXI can potentially be used for hyperhidrosis and the onset of effect is approximately 2 days compared to BoNT-A which can take up to a week for it to take full effect12.
Considering DAXI being so efficacious, why doesn’t everyone switch to DAXI then? The answer to this question is simple, because DAXI is more expensive compared to BoNT-A. Moreover, those who do not like the injection pattern or effects post injection would need to wait longer for the effects to wear off since the treatment effects of DAXI are not reversible immediately20. In addition, DAXI is not readily available and real-world data on its therapeutic effects and patient safety is still very limited. The indications for DAXI use are also limited, therefore,only time will tell if DAXI is a commercial success or failure. In conclusion, neuromodulators are important part of cosmetic business since they generate significant revenue, and their use will likely increase with the ageing population who wish to retain their youthful looks.
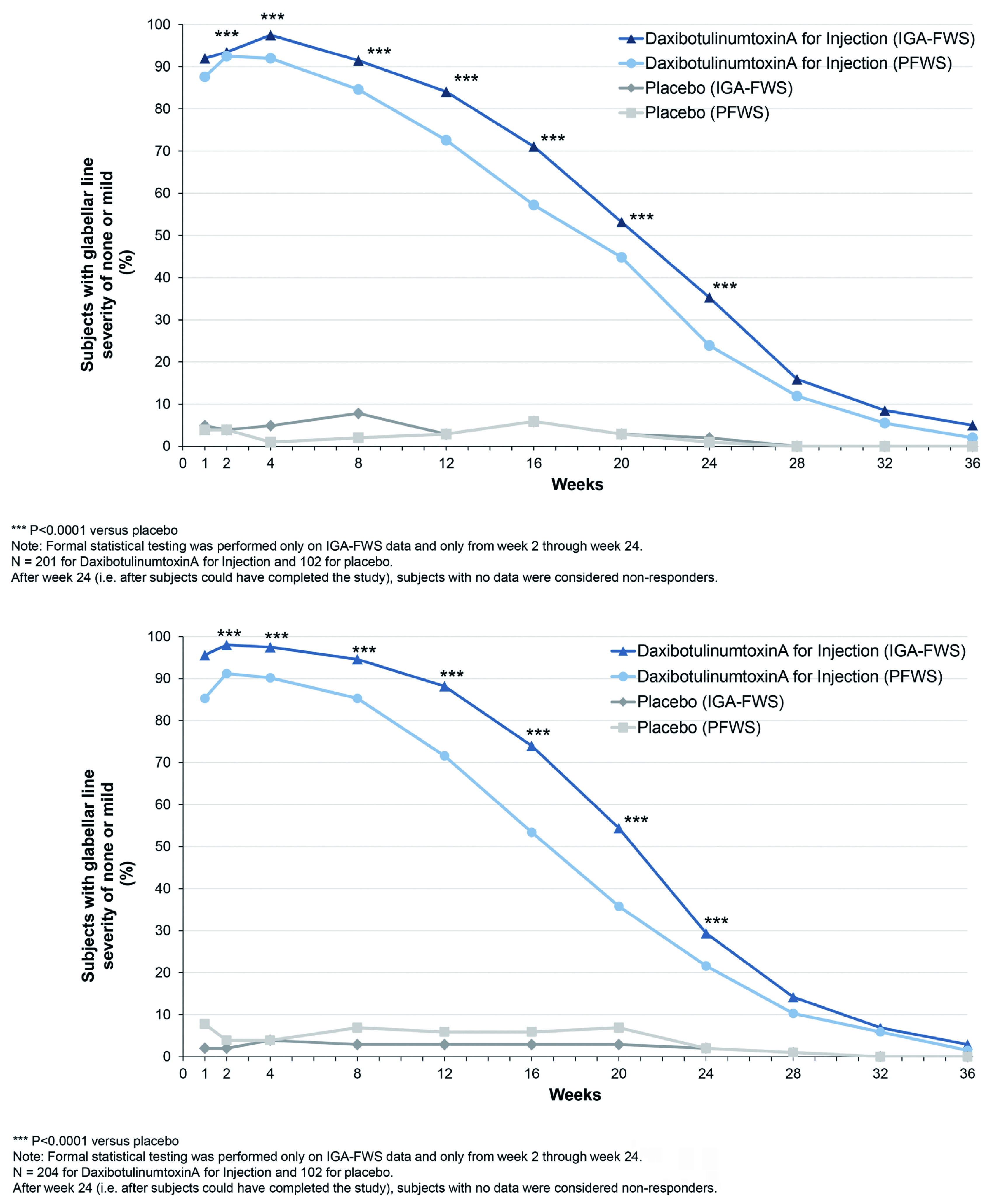
Figure 2. Proportion of subjects on DAXI vs placebo, over 36 weeks, rated 0 or 1 (none or mild) by investigator, 0 or 1 by the subject, and 0 or 1 with at least a 2-point improvement from their baseline based on both investigator and subject rating13.
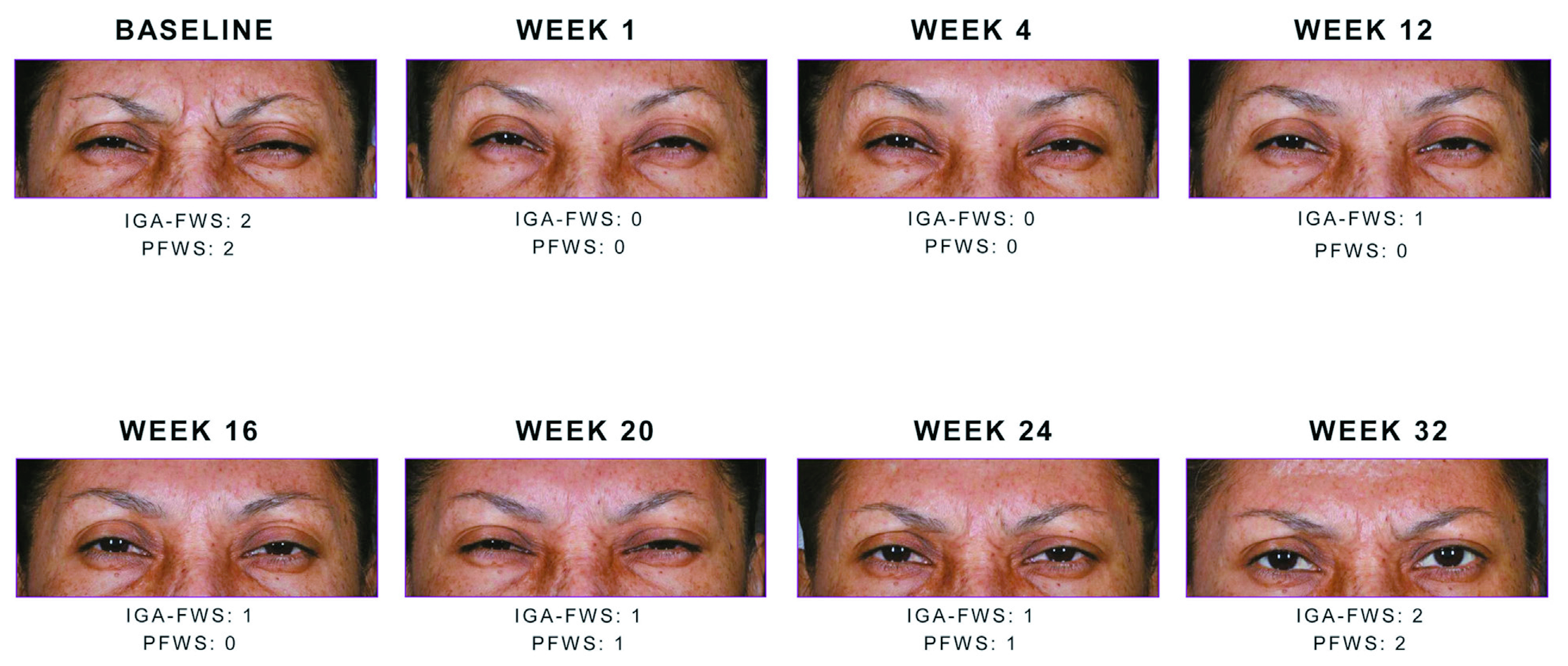
Figure 3. Study participants showing a 2-point reduction in glabellar line severity at maximum frown between weeks 1 to week 32. Note the sustained reduction with Daxi throughout week20.
References
1. Suozzi KC, et al. J Am Acad Dermatol 2022; 86(2): 463-7. 2. Satriyasa BK, et al. Clin Cosmet Investig Dermatol 2019; 12: 223-8. 3. Trindade De Almeida AR, et al. Dermatol Surg 2011; 37(11): 1553-65. 4. Awan KH, et al. Saudi Pharm J 2017; 25(1): 18-24. 5. Padda IS, et al,. Treasure Island (FL): StatPearls Publishing LLC.; 2022. 6. Carruthers J, et al. Facial Plast Surg Clin North Am 2007; 15(1): 51-4, vi. 7. Sneath J, et al. Dermatol Surg 2015; 41 Suppl 1: S82-7. 8. Multani I, et al. Pediatric Drugs 2019; 21(4): 261-81. 9. Shtefan V, et al. Clin Cosmet Investig Dermatol 2022; 15: 1045-9. 10. Torres S, et al. Clin Cosmet Investig Dermatol 2014; 7: 11-7. 11. Fabbri M, et al. Neurotox Res 2016; 29(1): 105-17. 12. Solish N, et al. Drugs 2021; 81(18): 2091-101. 13. Carruthers JD, et al. Plast Reconstr Surg 2020; 145(1): 45-58. 14. Solish N, et al. Aesthet Surg J 2022. 15. de la Torre BG, et al. Molecules 2023; 28(3). 16. Green JB, et al. Dermatol Surg 2021; 47(1): 42-6. 17. Carruthers A, et al. Dermatol Surg 2005; 31(4): 414-22; discussion 22. 18. Dover JS, et al. Dermatol Surg 2023; 49(1): 60-5. 19. Nestor MS, et al. J Cosmet Dermatol 2020; 19(11): 2785-804. 20. Bertucci V, et al. Journal of the American Academy of Dermatology 2020; 82(4): 838-845.





